- Visibility 257 Views
- Downloads 49 Downloads
- Permissions
- DOI 10.18231/j.ijnmhs.2024.020
-
CrossMark
- Citation
Metabolic dysfunction-associated steatotic liver disease: a narrative review of pathophysiology, diagnosis, and management
Abstract
Metabolic Dysfunction-Associated Steatotic Liver Disease(MASLD) poses a significant healthcare burden, affecting approximately 38% of the global population. The rising prevalence of MASLD, particularly among younger individuals, increases the risk of severe liver complications such as cirrhosis and hepatocellular carcinoma. In this narrative review, we present a detailed examination of MASLD, previously referred to as Non-alcoholic Fatty Liver Disease(NAFLD), which is distinguished by the accumulation of excess fat in hepatocytes without the involvement of alcohol intake. We examine the multifaceted pathophysiology of MASLD, showing the interplay of metabolic, genetic, and environmental factors contributing to its development and progression. Diagnostic approaches are discussed, which show the role of non-invasive imaging techniques such as ultrasound, CT, and MRI, alongside histopathological evaluation when necessary. The review also explores the potential of biomarkers related to inflammation, fibrosis, and oxidative stress in improving diagnostic accuracy and monitoring disease progression. Management strategies for MASLD focus mainly on lifestyle adjustments, such as changes in diet, enhanced physical activity, and weight reduction, which are vital for improving liver steatosis and preventing the progression of the disease. Additionally, pharmacological treatments targeting various pathophysiological pathways, such as insulin resistance and lipid metabolism, are reviewed. Promising agents include pioglitazone, GLP-1 receptor agonists, SGLT2 inhibitors, resmetirom, FGF21 analogues, and lanifibranor. This review highlights the need for continued research into the factors influencing MASLD to develop individualized prevention and treatment strategies. By summarizing current knowledge and identifying future research directions, this narrative review aims to contribute to the better understanding and management of MASLD, ultimately reducing its global health burden.
Introduction
Metabolic dysfunction-associated steatotic liver disease(MASLD), previously known as non-alcoholic fatty liver disease(NAFLD), is marked by the buildup of excess fat in liver cells, occurring independently of alcohol use. While the presence of a minimal amount of fat in the liver is physiologically normal, a hepatic fat content exceeding 5% to 10% of the liver's total weight is indicative of steatosis. The progression of MASLD can lead to a more severe condition termed metabolic dysfunction-associated steatohepatitis (MASH), previously referred to as non-alcoholic steatohepatitis (NASH). MASH is marked by hepatic inflammation and cellular injury, which can result in significant liver damage and fibrosis. [1]
Although only a small fraction of individuals with MASLD will advance to cirrhosis or hepatocellular carcinoma, the large affected population results in a growing number of patients at risk for these severe complications. MASLD has swiftly emerged as the most prevalent liver disease worldwide, currently estimated to impact 38% of the global population. Alarmingly, MASLD is being diagnosed at younger ages, thereby increasing the likelihood of developing significant complications over time. [2]
The challenge in addressing MASLD lies in its multifaceted pathophysiology, which involves a complex interplay of metabolic, genetic, and environmental factors, making it difficult to develop effective, universally applicable treatments and prevention strategies.[3] The diagnosis of MASLD involves a combination of clinical assessment, imaging techniques, and sometimes histopathological evaluation. Non-invasive imaging methods, such as ultrasound, CT, and MRI, are commonly employed to detect hepatic steatosis, while transient elastography(FibroScan) is increasingly used to assess liver fibrosis. Despite being the gold standard for diagnosing MASH and staging fibrosis, the invasive nature of liver biopsy limits its widespread use. [4]
Management strategies for MASLD focus primarily on lifestyle modifications, including dietary changes, increased physical activity, and weight loss, which are crucial for improving hepatic steatosis and preventing disease progression. Pharmacological treatments targeting various pathophysiological pathways are under investigation, with some showing promise in clinical trials. Additionally, the potential role of supplements and alternative therapies in managing MASLD is being explored. [5]
This narrative review seeks to offer an in-depth overview of the current knowledge on MASLD, covering its pathophysiology, diagnostic methods, and management approaches. It also highlights the associated comorbidities and complications and discusses future directions in research and treatment.
Discussion
We performed an extensive search across multiple databases using pertinent keywords, with no restrictions on publication date. Only papers published in English were considered. Additionally, grey literature was considered to ensure a thorough review of available evidence. This approach aimed to capture all pertinent studies related to Metabolic Dysfunction-Associated Steatotic Liver Disease, including various aspects of its pathophysiology, diagnosis, and treatment.
Pathophysiology of MASLD
Metabolic dysfunction-associated steatotic liver disease develops through a complex interplay of metabolic, genetic, and environmental factors. The pathogenesis is often described by the "multiple-hit" hypothesis, which suggests that multiple factors act synergistically to induce hepatic steatosis, inflammation, and fibrosis. [6]
Mechanisms of hepatic steatosis
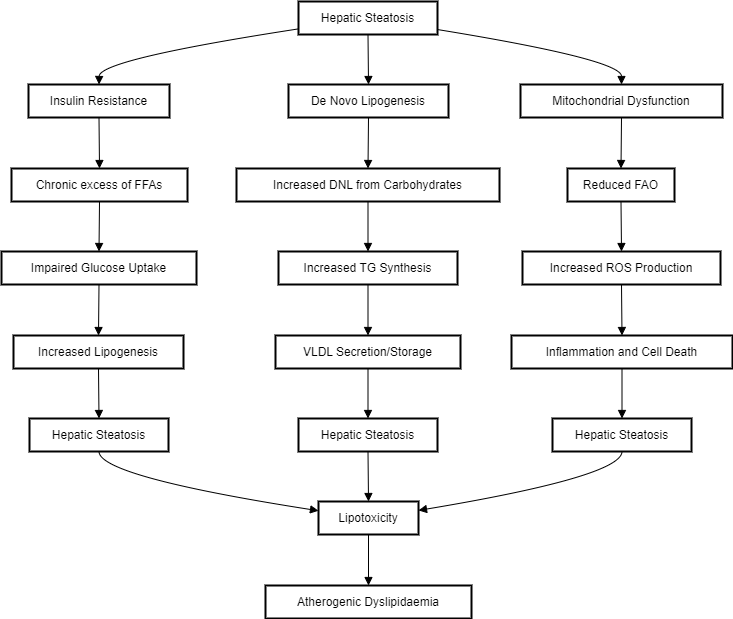
Under normal conditions, the liver does not store triacylglycerol(TG); however, during stressed states such as obesity or high fat/high carbohydrate intake, alcohol consumption, abnormal lipid metabolism results in ectopic hepatic lipid accumulation. This condition, known as hepatic steatosis, represents the common final endpoint. [7] Hepatic lipid levels are regulated by the balance between lipid acquisition and disposal, encompassing the four main pathways of hepatic lipid homeostasis. The liver acquires lipids via the uptake of circulating fatty acids and de novo lipogenesis. On the other hand, lipids are disposed of through oxidation(in mitochondria, peroxisomes, and cytochromes) and export as very low-density lipoprotein (VLDL) particles. Therefore, lipid accumulation occurs when lipid acquisition pathways surpass disposal pathways.[8] ([Figure 1])
Insulin Resistance - Insulin resistance is a main feature in the development of MASLD. Under normal physiological states, during fasting, low insulin levels promote the breakdown of fat from adipose tissue, providing energy for muscles, and stimulate the liver to produce glucose. After eating, insulin levels rise, inhibiting fat breakdown and promoting glucose uptake by muscles and the liver.[9] In insulin-resistant states like obesity, MASLD, and type 2 diabetes, this metabolic flexibility is disrupted, leading to a chronic excess of free fatty acids(FFAs) in the liver and muscles, a condition known as lipotoxicity. This excess fat, combined with impaired glucose uptake and increased lipogenesis in the liver, results in hepatic steatosis. The liver attempts to manage FFAs through oxidation, re-esterification into TG, and export as VLDL particles. Over-secretion of VLDL cholesterol in insulin-resistant individuals contributes to atherogenic dyslipidaemia. [10]
De Novo Lipogenesis - We need to first understand the TG metabolism in MASLD. Fatty acids, which are essential for TG synthesis, come from three main sources under normal conditions. First, dietary TGs are transported to hepatocytes for breakdown, aided by chylomicron remnants after partial absorption. Second, de novo lipogenesis(DNL) converts carbohydrates into FA. Third, FA are released into circulation as FFA through tissue mobilization. These FA can either be oxidized or synthesized into TGs within the liver. The hepatic TGs are then either excreted as very VLDL or stored in the liver. [11] DNL is the biochemical process in which fatty acids are synthesized from acetyl-CoA subunits, typically derived from carbohydrate catabolism. It has been suggested that DNL is abnormally elevated and contributes to the pathogenesis of MASLD. Additionally, DNL is increased under conditions of insulin resistance like type 2 diabetes mellitus. [12]
Mitochondrial oxidative function - Contrary to previous understanding, not all studies agree that mitochondrial oxidative function is impaired in simple steatosis and MASH. For instance, liver mitochondria from obese mice with hepatic steatosis showed no defects in respiratory function and even increased fatty acid oxidation. [13] However, perfused livers and isolated hepatocytes from obese rats with fatty liver exhibited decreased mitochondrial FAO. This suggests that fatty liver may not be due to primary mitochondrial damage but rather to altered regulation of mitochondrial FAO in hepatocytes.[14] The reduction in FAO in steatotic perfused livers is believed to result from the inhibition of carnitine palmitoyl-transferase 1(CPT1), which regulates the entry of long-chain fatty acids into the mitochondria. Elevated glycolysis in obesity leads to increased malonyl-CoA, a CPT1 inhibitor, causing steatosis by blocking CPT1 and FAO in the liver. When mitochondria are isolated, the malonyl-CoA is diluted, explaining the observed increase in FAO. Reducing malonyl-CoA synthesis or decreasing CPT1 sensitivity to its inhibition could prevent steatosis. [15] In humans with simple steatosis and insulin resistance, mitochondrial FAO and respiratory function are elevated, even after isolating mitochondria from liver biopsies. This indicates that the changes in mitochondrial function seen in MASLD are due to a change in mitochondrial composition, possibly as an adaptation to increased demands for ATP and TCA cycle intermediates from gluconeogenesis and lipid handling. MASLD is associated with increased hepatic glucose production and lipid storage, raising mitochondrial ATP demand and oxidative capacity. This adaptation helps prevent free fatty acid toxicity in hepatocytes but also increases ROS production, leading to inflammation, impaired insulin signalling, and cell death. Restoring mitochondrial fuel preference might be a therapeutic target for MASLD, as increased TCA cycle flux and impaired ketogenesis contribute to hepatic steatosis and hyperglycaemia. [16]
Role of inflammation and fibrosis progression
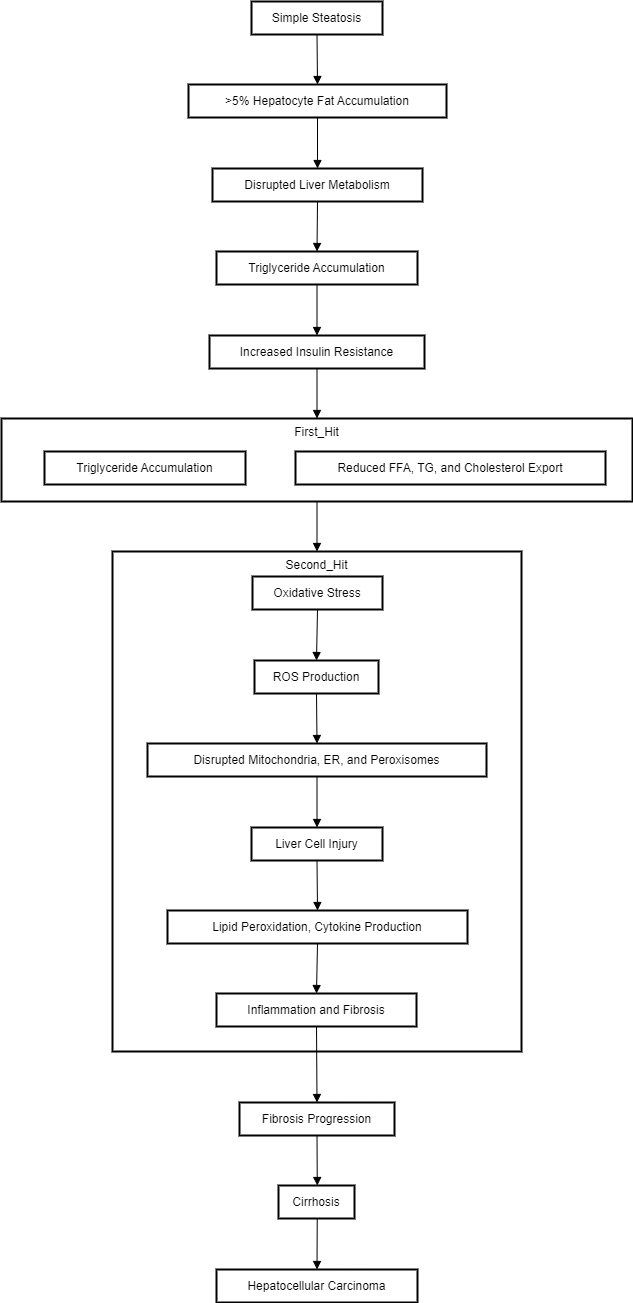
MASLD progresses from simple steatosis to steatohepatitis, fibrosis, cirrhosis, and eventually hepatocellular carcinoma due to insulin resistance, oxidative stress, and lipotoxicity caused by a long-term Western diet. Simple hepatic steatosis, the initial stage of MASLD, involves minimal fat deposition without immune cell infiltration or liver cell damage. When fat accumulates in more than 5% of hepatocytes, it disrupts liver metabolism. [17]
Day and James [18] proposed the widely accepted "two-hit" theory to explain the progression from MASLD to MASH. The "first hit" is characterized by simple steatosis, where increased intake of free fatty acids leads to triglyceride accumulation in hepatocytes and increased insulin resistance. Additionally, the export of free fatty acids, triglycerides, and cholesterol is significantly reduced, constituting the "first hit." The "second hit" is initiated by oxidative stress, which further disrupts metabolic processes in mitochondria, the endoplasmic reticulum, and peroxisomes. Reactive oxygen species inhibit mitochondrial enzymes, inactivate glyceraldehyde-3-phosphate dehydrogenase, and disrupt membrane sodium channels, leading to liver cell injury. ROS also exacerbates lipid peroxidation, cytokine production, and lipid accumulation, promoting inflammation and fibrosis through various protein kinases and nuclear transcription factor activation pathways.([Figure 2])
Genetic and epigenetic factors
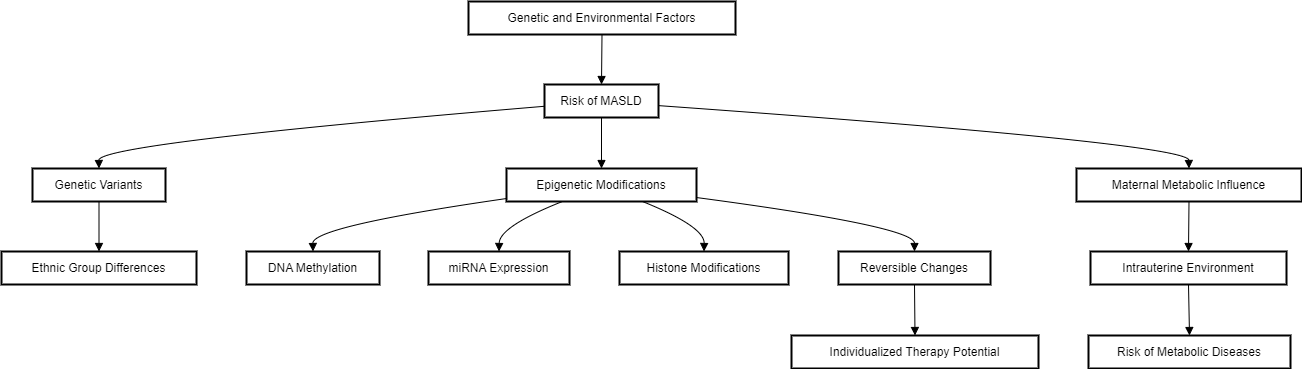
The risk and severity of MASLD development are influenced by the interactions between genetic and environmental factors, including diet and physical activity. Genome-wide association studies(GWAS) and candidate gene approaches have identified several genetic variants linked to MASLD and/or MASH. Genetics also explains variations in MASLD prevalence among different ethnic groups; for example, Hispanics have a particularly high prevalence, while Blacks have the lowest, partly due to differences in the occurrence of single. [19]
The metabolic phenotype of the mother and the intrauterine environment play significant roles in the development of metabolic diseases later in life. Beyond genetic predisposition, epigenetic changes in response to environmental factors like nutrition also contribute to disease risk. Epigenetic modifications, which alter gene expression and phenotype, have been linked to MASLD in various mouse and human studies. These include changes in DNA methylation patterns, miRNA expression, and histone modifications. Since epigenetic changes are both inheritable and reversible, they present new opportunities for individualized prevention and therapy.[19] ([Figure 3])
Diagnosis of NAFLD
The diagnosis of MASLD typically involves a combination of clinical evaluation, imaging techniques, and biochemical markers. While non-invasive techniques are becoming more common, liver biopsy is the gold standard for diagnosing MASH and assessing the stage of fibrosis.
Medical history and physical examination
Patients with MASLD are often asymptomatic, but when symptoms do occur, they may include right upper quadrant pain, jaundice, and pruritus. It is essential to rule out common causes of liver injury such as alcohol and drug use. A thorough history should include an exploration of diet, physical activity, weight changes, and an assessment for associated conditions like diabetes, hypertension, hyperlipidaemia, obesity, and sleep apnoea. Physicians should also assess risk factors for viral hepatitis, including intravenous drug use, blood transfusion, and sexual activities. A patient's family history should be reviewed for cardiovascular and metabolic disorders, as well as chronic liver disease. It may also be necessary to evaluate for hereditary or less common conditions that could initially present with abnormal liver tests. Vital signs should be recorded, including blood pressure, weight, BMI, and waist circumference. Although the physical examination is often unremarkable, it may reveal elevated blood pressure, central obesity, and hepatosplenomegaly. [20]
Laboratory tests
No single biochemical marker can definitively diagnose MASLD or differentiate between its stages, such as steatosis, MASH, and cirrhosis. While mildly elevated serum aminotransferase levels are common in MASLD patients, liver enzymes can be normal in up to 78% of cases.[21] The entire spectrum of MASLD can occur even with normal alanine aminotransferase(ALT) levels, making liver enzyme levels unreliable for diagnosis. When present, ALT and aspartate aminotransferase (AST) elevations are typically mild and usually not more than four times the upper limit of normal. [22]
Serum gamma-glutamyltransferase(GGT) is often elevated in MASLD and associated with increased mortality and advanced fibrosis. Elevated GGT activity could indicate a higher utilization of glutathione, an intracellular antioxidant, serving as a marker of oxidative stress. Consequently, increased GGT activity may signify oxidative stress in individuals with MASLD. [23]
In MASH, elevated ferritin levels occur in up to 50% of patients, and increased transferrin saturation in about 10%, although these do not correlate with liver iron concentration, and their role in MASH pathogenesis is unclear.[24] In cirrhosis, which is the end of the spectrum, hypoalbuminemia, hyperbilirubinemia, thrombocytopenia, and prolonged prothrombin time can be observed. [21]
Since these markers don’t differentiate the prognosis and stage of MASLD, current research focuses on biomarkers related to key mechanisms of MASH pathogenesis, including inflammation, fibrosis, oxidative stress, and hepatocyte apoptosis.
A study (Duan et al. 2022) [25] on the relationship between inflammatory markers and MASLD in obese children showed that, plasma levels of IL-1β, IL-6, IL-8, IL-12, IL-17, IL-21, IL-32, and TNF-α were elevated in obese children with MASLD compared to those with simple obesity. It also showed that these markers were more elevated in MASH compared to MASLD, which helps identify the progression of disease. It emphasizes the role of inflammatory cytokines, such as IL-1β, IL-6, and notably IL-17, in the development and progression of MASLD. These cytokines are involved in various inflammatory pathways that interfere with insulin signalling, contributing to liver inflammation and fibrosis. Furthermore, the study suggests that SIRT1, a regulator of inflammation, may be involved in the regulation of inflammatory cytokines in MASLD and could be a critical factor in the disease's progression. [25]
As the disease progresses, it moves towards fibrosis. Liver biopsy is the gold standard for diagnosis, however as medicine moves towards a non-invasive age, the need for blood investigations becomes important. Type IV collagen(cIV) is a promising low-cost serum biomarker for identifying significant and advanced fibrosis in MASLD patients. A study by (Stefano et al. 2021)[26] showed that patients with cIV levels above 30 ng/mL had a 5.57-fold higher chance of having significant fibrosis compared to those with lower levels. Other biomarkers such as hyaluronic acid(HA), procollagen III (PC III), laminin(LN), and collagen glycoprotein (CG) were not significantly related to significant fibrosis after adjusting for other variables. cIV demonstrated an ability to discriminate between stages of fibrosis. cIV could be used in routine clinical practice as a simple and cost-effective tool to predict significant and advanced fibrosis in MASLD patients, potentially guiding the need for liver biopsy. [26]
As we have already seen in the pathogenesis section, oxidative stress is a major contributor in MASLD and its progression to MASH. A study by(Zhu et al. 2023)[27] showed that the Trx system, which includes thioredoxin (Trx), thioredoxin reductase(TrxR), and nicotinamide adenine dinucleotide phosphate(NADPH), has a critical role in maintaining cellular redox homeostasis by reducing oxidized proteins and scavenging ROS. In response to the increased oxidative stress in MASH, cells upregulate the expression and activity of Trx to counteract the damaging effects of ROS. Elevated Trx levels in the serum of MASH patients have been identified, making it a potential biomarker for distinguishing MASH from simple steatosis. Higher Trx levels correlate with the severity of oxidative stress and liver damage, reflecting the body's efforts to neutralize the harmful effects of ROS. [27]
A study by(Zhang et al. 2023) [28] spoke about the utility of measuring cytokeratin-18 M30(CK-18 M30) levels as a non-invasive diagnostic tool for MASH. Cytokeratin-18(CK-18) is a protein that forms part of the intermediate filament structure within epithelial cells, particularly hepatocytes. During cell apoptosis, CK-18 is cleaved by caspases, are released into the bloodstream. One of these fragments is known as CK-18 M30.
CK-18 M30 can be measured in the blood, and elevated levels are indicative of increased hepatocyte apoptosis, a feature common in liver diseases such as MASH. Data from 14 centres showed that CK-18 M30 levels were higher in MASH patients compared to those with MASLD. [28]
Imaging in the diagnosis of MASLD
Imaging techniques play a crucial role in the diagnosis of MASLD, providing non-invasive methods to detect and quantify hepatic steatosis and fibrosis. These advanced imaging modalities are essential for early detection, monitoring disease progression, and guiding treatment strategies.
Ultrasound - Ultrasound is often the first-line imaging modality used for the detection of hepatic steatosis due to its wide availability, non-invasiveness, and cost-effectiveness. Transabdominal ultrasound is the primary imaging method for suspected MASLD, which helps monitor both steatosis and fibrosis. Steatosis is graded from 0(normal) to 3 (severe). B-mode ultrasound is effective for detecting moderate to severe steatosis but has low sensitivity for mild cases. Newer methods like the controlled attenuation parameter(CAP) and quantitative ultrasound techniques offer improved detection, especially for moderate to severe steatosis. However, these methods still face challenges in accurately identifying mild steatosis. Optimal detection methods are still under development. [29] Liver fibrosis degree is crucial for prognosis and mortality in MASLD. Advanced fibrosis increases liver-related events, while cirrhosis mostly leads to liver-related events. Histology remains the gold standard but is invasive. Non-invasive methods like sonography and elastography are preferred. Sonography shows advanced liver changes but has limited sensitivity for early fibrosis. Transient elastography(TE), commonly known by its commercial name Fibro Scan, measures liver stiffness via shear wave speed, correlating well with fibrosis stages. Point shear wave elastography (pSWE) and 2D shear wave elastography (2D-SWE) use acoustic radiation force, offering improved diagnostic accuracy, especially for advanced fibrosis. However, TE's and 2D-SWE's accuracy varies with liver disease etiology, and high BMI can hinder results. Despite their advantages, elastography methods face limitations like technical failure rates and device availability. [29]
Computed Tomography(CT) - CT imaging, particularly unenhanced CT, is effective in quantifying liver fat content by measuring attenuation values. The capacity to block x-rays is known as attenuation. In imaging, structures with high attenuation appear brighter, while those with low attenuation appear darker. Attenuation is depicted both by the grayscale appearance in the image and a numerical value known as the Hounsfield unit(HU). Higher HU values indicate greater x-ray blockage. Therefore, a high attenuation structure, such as bone, will have a higher HU value compared to low attenuation structures like fat or air. Normal liver parenchyma has an attenuation of about 60 HU, while steatosis reduces this to around 40 HU. Using a threshold of 48 HU, unenhanced CT shows high specificity (100%) for severe steatosis (~30%) with a sensitivity of 54%. [30]
Dual Energy CT(DECT) uses two energy levels to better differentiate tissue compositions, enhancing detection of hepatic steatosis despite its limited advantage over conventional CT. Attenuation measurements from virtual non-contrast(VNC) CT correlate well with liver fat content, providing high specificity for diagnosing hepatic steatosis. [31]
Photon-counting CT(PCCT) shows great promise in the diagnosis and management of MASLD. PCCT detectors offer a unique advantage over conventional CT detectors by measuring single photons and their energy. In the context of MASLD, the enhanced material decomposition of PCCT allows for better differentiation between liver tissues and fat deposits. The ability to measure single photons and their energy enables PCCT to detect subtle differences in tissue composition that conventional CT might miss. This precision is particularly important for identifying and quantifying hepatic steatosis, a key characteristic of MASLD. [32]
Future advancements include deep learning-based automatic assessment of CT liver HU values, enhancing reproducibility and objectivity. Automated algorithms can improve measurement accuracy and avoid human bias. [30]
Magnetic Resonance Imaging(MRI) - MRI techniques for detecting and quantifying liver fat content have advanced from qualitative methods to more accurate quantitative techniques such as MR spectroscopy(MRS) and chemical shift-encoded MRI(CSE-MRI). Conventional qualitative MRI methods, while useful for evaluating steatosis, lack the accuracy needed for quantitative assessments due to various confounding factors. Quantitative methods like CSE-MRI and MRS exploit the chemical shift between fat and water protons to measure liver fat content accurately. The proton density fat fraction (PDFF), derived from these measurements, is expressed as a percentage and correlates well with histologic fat content. PDFF is increasingly recognized as the best technique for quantifying liver fat, surpassing even biopsies in some aspects. [30]
MRS provides high-resolution spectra to distinguish fat and water peaks, while CSE-MRI offers real-time PDFF mapping across the liver. Both methods have shown high reproducibility and accuracy, with CSE-MRI also allowing for simultaneous assessment of iron deposition in the liver. Future improvements include automation of measurement processes and reduction of acquisition time using advanced techniques like compressed sensing and MR fingerprinting. These advancements aim to enhance the efficiency and accuracy of liver fat quantification in clinical settings. [30]
Liver biopsy
Percutaneous liver biopsy remains the gold standard for differentiating isolated fatty liver from MASH. The key histopathologic features of MASH include macrovesicular steatosis, lobular inflammation, hepatocyte ballooning, and frequently perisinusoidal/perivenular fibrosis along with Mallory–Denk bodies. However, pathologists do not always agree on these criteria. The NAFLD activity score(NAS), developed by the NASH Clinical Research Network sponsored by the National Institutes of Health, is a histologic scoring system that evaluates steatosis, lobular inflammation, and hepatocyte ballooning. A NAS of 5 or more indicates MASH, while a score of 2 or less suggests the absence of MASH, with scores of 3 or 4 being indeterminate. The NAS is designed to uniformly assess disease severity, particularly in clinical trials, rather than serve as a diagnostic tool. [22]
Treatment of MASLD
The management of MASLD focuses on lifestyle modifications, pharmacological treatments, and emerging therapies to address the multifaceted nature of the disease. Treatment aims to reduce liver fat, improve insulin sensitivity, and prevent the progression to more severe forms of liver disease such as MASH, cirrhosis, or HCC.
Lifestyle modification in MASLD
Lifestyle modifications are crucial in the management of metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis. Key interventions include dietary changes, increased physical activity, and weight loss. Studies have demonstrated that even modest weight loss(5-10% of body weight) can significantly improve liver steatosis, inflammation, and fibrosis. Dietary modifications should focus on reducing caloric intake, particularly from fats and sugars, and incorporating more fruits, vegetables, and whole grains. [33]
Physical activity, including both aerobic and resistance exercises, helps reduce liver fat independently of weight loss and is essential for overall metabolic health. Regular exercise also helps in managing comorbid conditions like type 2 diabetes and cardiovascular diseases, which are often associated with MASLD. Despite the proven benefits, sustained lifestyle changes are challenging for many patients, and adherence can be low. Therefore, personalized approaches and support from healthcare professionals are vital to help patients implement and maintain these lifestyle changes for long-term health benefits. [33]
Pharmacological treatments
While lifestyle modifications are essential, pharmacological treatments can provide additional benefits, particularly in patients with significant fibrosis or those who fail to achieve sufficient lifestyle changes.
Pioglitazone - Pioglitazone is a thiazolidinedione(TZD) class drug used primarily in the treatment of type 2 diabetes mellitus. Pioglitazone binds to the peroxisome proliferator-activated receptor-gamma (PPAR-γ), which is a nuclear receptor found predominantly in adipose tissue, as well as in muscle and liver. Activation of PPAR-γ leads to the transcription of various insulin-responsive genes involved in glucose and lipid metabolism. This is of interest in MASLD as insulin sensitivity will reduce hepatic steatosis. [34]
A meta-analysis by(Zhao et al. 2022)[35] evaluates the efficacy of pioglitazone in treating MASH. Researchers analysed 15 randomized controlled trials(RCTs) and concluded that the efficacy of pioglitazone was 78% higher than that of the control group (risk ratio = 1.78, 95% CI: 1.31–2.43).
GLP-1 Receptor Agonists - Like pioglitazone, GLP-1 receptor agonists are also predominantly used in the treatment of type 2 diabetes mellitus. GLP-1 receptor agonists stimulate insulin release from the pancreatic beta cells in a glucose-dependent manner. They also suppress the secretion of glucagon from pancreatic alpha cells, ultimately improving glycaemic control.[36] GLP-1 receptor agonists show promise in treating MASLD by enhancing insulin sensitivity, reducing liver fat accumulation, and decreasing inflammation and oxidative stress. Their effects are partly independent of weight loss, involving multiple direct and indirect pathways. Despite the strong preclinical and clinical evidence, the precise mechanisms and the presence of GLP-1 receptors in liver cells remain controversial and require further investigation. [37]
SGLT2 Inhibitors - SGLT2 proteins are primarily located in the proximal convoluted tubules of the kidneys. These proteins are responsible for reabsorbing the glucose filtered by the kidneys back into the bloodstream. By inhibiting SGLT2, these drugs prevent glucose reabsorption in the proximal tubules. [38] SGLT-2 inhibitors regulate processes such as endoplasmic reticulum stress, oxidative stress, inflammation, autophagy, and apoptosis, which are implicated in MASLD pathogenesis. They have shown beneficial effects on MASLD in vitro, in animal models, and in clinical trials, particularly among patients with type 2 diabetes mellitus. [39]
Resmetirom - It is a thyroid hormone receptor beta(TRβ) agonist, has shown promise in clinical trials by significantly reducing liver fat and improving metabolic parameters. Its mechanism involves enhancing fatty acid oxidation and reducing lipogenesis in the liver, thus directly targeting the hepatic fat accumulation seen in MASLD. Early-phase trials have demonstrated its potential in resolving MASH and reducing fibrosis. [40]
Fibroblast Growth Factor 21(FGF21) Analogs - FGF21 analogs, such as pegbelfermin, target multiple pathways involved in MASLD, including lipid metabolism, insulin sensitivity, and inflammation. Clinical trials have shown that these agents can reduce liver fat content and improve liver histology. Their broad metabolic effects make them promising candidates for treating MASLD, particularly in advanced stages of the disease. [40]
Lanifibranor - It is a pan-PPAR agonist, acts on PPARα, PPARγ, and PPARδ receptors, addressing various aspects of MASLD pathophysiology, including insulin resistance, inflammation, and fibrosis. Clinical trials have shown improvements in liver enzymes, lipid profiles, and liver histology, making it a potential comprehensive treatment for MASLD. [40]
Conclusion
In conclusion, Metabolic Dysfunction-Associated Steatotic Liver Disease, represents a significant and growing global health challenge due to its widespread prevalence and potential to progress to severe liver conditions, including cirrhosis and hepatocellular carcinoma. The complex interplay of metabolic, genetic, and environmental factors underlying MASLD necessitates a multifaceted approach to diagnosis and treatment. Advances in non-invasive imaging techniques and biomarkers have improved diagnostic accuracy and patient monitoring. Effective management strategies emphasize lifestyle modifications, such as dietary changes and increased physical activity, to reduce hepatic fat and improve overall metabolic health. Pharmacological treatments targeting various pathophysiological pathways show promise in clinical trials, offering hope for more effective therapies. Future research should continue to explore these therapeutic avenues and the role of genetic and epigenetic factors to develop individualized prevention and treatment strategies, ultimately reducing the burden of MASLD on global health.
Source of Funding
None.
Conflict of Interest
None.
References
- Rivera W. Nonalcoholic Fatty liver Disease (NAFLD). . 2024. [Google Scholar]
- Wong V, Ekstedt M, Wong G, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79(3):842-52. [Google Scholar]
- Carr R, Oranu A, Khungar V. Nonalcoholic Fatty Liver Disease. Gastroenterol Clin Am . 2016;45(4):639-52. [Google Scholar]
- Ajmera V, Loomba R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol Metab. 2021;50. [Google Scholar]
- Kwak MS, Kim D. Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Korean J Int Med/Korean J Int Med. 2018;33(1):64-74. [Google Scholar]
- Huang G, Wallace D, Powell E, Rahman T, Clark P, Subramaniam V. Gene Variants Implicated in Steatotic Liver Disease: Opportunities for Diagnostics and Therapeutics. Biomedicines. 2023;11(10). [Google Scholar]
- Nassir F, Rector R, Hammoud G, Ibdah J. Pathogenesis and prevention of hepatic steatosis. Gastroenterol Hepatol (N Y). 2015;11(3):167-75. [Google Scholar]
- Ipsen D, Lykkesfeldt J, Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. . Cell Mol Life Sci. 2018;75(18):3313-40. [Google Scholar]
- Petersen M, Shulman G. Mechanisms of Insulin Action and Insulin Resistance. Physiological Rev. 2018;98(4):2133-223. [Google Scholar]
- Nogueira J, Cusi K. Role of Insulin Resistance in the Development of Nonalcoholic Fatty Liver Disease in People With Type 2 Diabetes: From Bench to Patient Care. Diabetes Spectrum. 2024;37(1):20-8. [Google Scholar]
- Zhu Z, Zhang X, Pan Q, Zhang L, Chai J. In-depth analysis of de novo lipogenesis in non-alcoholic fatty liver disease: Mechanism and pharmacological interventions. Liver Res. 2023;7(4):285-95. [Google Scholar]
- Sanders F, Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev Biol Rev Cambridge Philosophical Soc. 2016;91(2):452-68. [Google Scholar]
- Brady L, Brady P, Romsos D, Hoppel C. Elevated hepatic mitochondrial and peroxisomal oxidative capacities in fed and starved adult obese (ob/ob) mice. Biochem J. 1985;231(2):439-83. [Google Scholar]
- Mccune S, Durant P, Jenkins P, Harris R. Comparative studies on fatty acid synthesis, glycogen metabolism, and gluconeogenesis by hepatocytes isolated from lean and obese Zucker rats. . Metab Clin Exp. 1981;30(12):90037-45. [Google Scholar]
- Henninger C, Clouet P, Danh H, Pascal M, Bezard J. Effects of fenofibrate treatment on fatty acid oxidation in liver mitochondria of obese zucker rats. Biochem Pharm. 1987;36(19):90638-]9. [Google Scholar]
- Shum M, Ngo J, Shirihai O, Liesa M. Mitochondrial oxidative function in NAFLD: Friend or foe?. Mol Metab. 2021;50. [Google Scholar]
- Ma Y, Lee G, Heo S, Roh Y. Oxidative Stress Is a Key Modulator in the Development of Nonalcoholic Fatty Liver Disease. Antioxidants. 2021;11(1). [Google Scholar]
- Day C, James O. Steatohepatitis: A tale of two “hits”? Gastroenterology. Gastroenterology. 1998;114(4):70599-601. [Google Scholar]
- Jonas W, Schürmann A. Genetic and epigenetic factors determining NAFLD risk. . Mol Metab. 2021;50. [Google Scholar]
- Wilkins T, Tadkod A, Hepburn I, Schade R. Nonalcoholic fatty liver disease: diagnosis and management. World J Hepatol. 2013;88:35-42. [Google Scholar]
- Obika M, Noguchi H. Diagnosis and Evaluation of Nonalcoholic Fatty Liver Disease. . Exp Diab Res. 2012;3(2):1-12. [Google Scholar]
- Torres D, Harrison S. Diagnosis and Therapy of Nonalcoholic Steatohepatitis. Gastroenterology. 2008;134(6):1682-98. [Google Scholar]
- Chen L, Huang M, Shyu Y, Chien R. Gamma-glutamyl transpeptidase elevation is associated with metabolic syndrome, hepatic steatosis, and fibrosis in patients with nonalcoholic fatty liver disease: A community-based cross-sectional study. Kaohsiung J Med Sci. 2021;37(9):819-46. [Google Scholar]
- Kowdley K, Belt P, Wilson L, Yeh M, Neuschwander B, Chalasani N. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55(1):77-85. [Google Scholar]
- Duan Y, Luo J, Pan X, Wei J, Li X. Association between inflammatory markers and non-alcoholic fatty liver disease in obese children. . Frontiers in Public Health. 2022;10. [Google Scholar]
- Stefano J, Guedes L, Souza D, Vanni A, Alves D, Carrilho V. Usefulness of collagen type IV in the detection of significant liver fibrosis in nonalcoholic fatty liver disease. . Ann Hepatol. 2021;20. [Google Scholar]
- Zhu M, Dagah O, Silaa BB, Lu J. Thioredoxin/Glutaredoxin systems and gut microbiota in NAFLD: interplay, mechanism, and therapeutical potential. Antioxidants. 2023;12(9):1680-1680. [Google Scholar]
- Zhang H, Rios R, Boursier J, Anty R, Chan W, George J. Hepatocyte apoptosis fragment product cytokeratin-18 M30 level and non-alcoholic steatohepatitis risk diagnosis: an international registry study. Chinese Med J Chin Med J. 2023;136(3):341-50. [Google Scholar]
- Petzold G. Role of Ultrasound Methods for the Assessment of NAFLD. J Clin Med. 2015;11(15). [Google Scholar]
- Jang W, Song J. Non-Invasive Imaging Methods to Evaluate Non-Alcoholic Fatty Liver Disease with Fat Quantification: A Review. Diagnostics. 2023;13(11). [Google Scholar]
- Xu J, Boesen M, Hansen S, Ulriksen P, Holm S, Lönn L. Assessment of Liver Fat: Dual-Energy CT versus Conventional CT with and without Contrast. Diagnostics. 2022;12(3). [Google Scholar]
- Hu N, Yan G, Tang M, Wu Y, Song F, Xia X. CT-based methods for assessment of metabolic dysfunction associated with fatty liver disease. European. 2023;7(1). [Google Scholar]
- Hallsworth K, Adams L. Lifestyle modification in NAFLD/NASH: Facts and figures. JHEP Rep. 2019;1(6):468-79. [Google Scholar]
- Smith U. Pioglitazone: mechanism of action. Int J Clin Pract Suppl. 2001;121:13-8. [Google Scholar]
- Zhao Y, Zhao W, Wang H, Zhao Y, Bu H, Takahashi H. Pioglitazone on nonalcoholic steatohepatitis: A systematic review and meta-analysis of 15 RCTs. Medicine. 2022;101(46). [Google Scholar]
- Collins L, Costello RA. Glucagon-Like peptide-1 receptor agonists. . 2024. [Google Scholar]
- Lee H, Kim H. Therapeutic mechanisms and clinical effects of glucagon-like peptide 1 receptor agonists in nonalcoholic fatty liver disease. Int J Mol Sci. 2023;24(11). [Google Scholar]
- Fonseca-Correa JI, Correa-Rotter R. Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. . Frontiers in Medicine. 2021;8. [Google Scholar]
- Androutsakos T, Nasiri-Ansari N, Bakasis A, Kyrou I, Efstathopoulos E, Randeva H. SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection. Int J Mol Sci. 2022;23(6). [Google Scholar]
- Ciardullo S, Muraca E, Vergani M, Invernizzi P, Perseghin G. Advancements in pharmacological treatment of NAFLD/MASLD: a focus on metabolic and liver-targeted interventions. Gastroenterology Rep. 2024;12:1-10. [Google Scholar]
- Abstract
- Introduction
- Discussion
- Pathophysiology of MASLD
- Mechanisms of hepatic steatosis
- Role of inflammation and fibrosis progression
- Genetic and epigenetic factors
- Diagnosis of NAFLD
- Medical history and physical examination
- Laboratory tests
- Imaging in the diagnosis of MASLD
- Liver biopsy
- Treatment of MASLD
- Lifestyle modification in MASLD
- Pharmacological treatments
- Conclusion
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Murugan V. Metabolic dysfunction-associated steatotic liver disease: a narrative review of pathophysiology, diagnosis, and management [Internet]. J Nutr Metab Health Sci. 2024 [cited 2025 Oct 10];7(3):110-118. Available from: https://doi.org/10.18231/j.ijnmhs.2024.020
APA
Murugan, V. (2024). Metabolic dysfunction-associated steatotic liver disease: a narrative review of pathophysiology, diagnosis, and management. J Nutr Metab Health Sci, 7(3), 110-118. https://doi.org/10.18231/j.ijnmhs.2024.020
MLA
Murugan, Vignesh. "Metabolic dysfunction-associated steatotic liver disease: a narrative review of pathophysiology, diagnosis, and management." J Nutr Metab Health Sci, vol. 7, no. 3, 2024, pp. 110-118. https://doi.org/10.18231/j.ijnmhs.2024.020
Chicago
Murugan, V.. "Metabolic dysfunction-associated steatotic liver disease: a narrative review of pathophysiology, diagnosis, and management." J Nutr Metab Health Sci 7, no. 3 (2024): 110-118. https://doi.org/10.18231/j.ijnmhs.2024.020
