- Visibility 1.4k Views
- Downloads 484 Downloads
- Permissions
- DOI 10.18231/j.ijnmhs.2020.021
-
CrossMark
- Citation
Antimicrobial activity of Phyllanthus niruri (Chanka piedra)
- Author Details:
-
Chandana G
-
Manasa R
-
Vishwanath S
-
Shekhara Naik R
-
Mahesh MS *
Abstract
Phyllanthus niruri Linnaus (Euphorbiaceae) also known as Chanka piedra is widely grown and used throughout the tropical and subtropical countries of the world. In India it is present in the coastal areas. It is an annual herb and field weed having very short life. Phyllanthus comprises of 600-700 species with minor distinguishing features among them. In Indian ayurvedic system, plant extract of P. niruri is used as a medicine for asthma, bronchitis, anemia, leprosy, etc., As mentioned in the book Charaka Samhita P. niruri is used as an effective treatment for stimulating liver, improving digestion, to increase appetite and produce laxative effects. Phyllanthus niruri is a traditional herb with long-standing Ayurvedic, Chinese and Malay ethnomedical records and antimicrobial activity. Antimicrobial activity refers to the process of killing or inhibiting the disease causing microbes. Methanolic extract of P. niruri is an effective antibacterial agent to treat bacterial infections since the extract exhibited significant antimicrobial potency. The alcoholic extract of leaves of Phyllanthus niruri shows significant antibacterial activity against cariogenic organisms. The anti microbial activity of aqueous extract is found to be more effective than the acetone extract of Phyllanthus niruri against pathogens responsible for common infections of skin, respiratory, urinary and gastro-intestinal tracts.
Introduction
With the increase in antibiotic resistance rates and the need for novel antibiotics, which have optimal antimicrobial activity with minimal toxicity, there is renewed interest in exploring phytochemicals from everyday plants. A possible reason for the increased interest in extracting phytochemicals for the development of novel antibiotics is the threat of plant species extinction, hence inciting a need to explore the medicinal potential of these resources before they are lost.[1] These include rutin,[2], [3] gallocatechin, [4] prenylated flavanone glycosides[5], quercetin [6], quercitrin,[7] p-Cymene,[8] corilagin,[9] diosgenin,[10] securinine[11] and -glucogallin.[12]
P. niruri is rich in several compounds which have antioxidant, anti-protozoal, anti-viral and anti-microbial activity [13]. Aqueous, alcholic, hydro-alcoholic and methanolic extracts of P. niruri has exhibited wide range of anti-microbial activity against Bacillus pumillus, Bacillus ceraus, E. coli, Vibrio cholera, Lactobacillus acidophilus (L. acidophilus), Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus), Coney lunata and Salmonella typhi, Listeria monocytogenes Helicobacter pylori (H. pylori) and Mycoplasma galisepticum.
Botanical classification: Phyllanthus niruri L.
Kingdom – Plantae
Division – Magnoliophyta
Class – Magnoliopsida
Order – Euphorbiales
Family – Euphorbiaceae
Genus – Phyllanthus
Species – Niruri
Vernacular names in India
Assamese: Holpholi; Poram-lokhi
Bengali: Noar
Hindi: Chalmeri, Harfarauri, Bhuiaonla.
Kannada: Kirunelli, Nela Nelli,
Konkani: Bhuin-avalae
Telugu: Ratsavusirike, Nela Usiri,
Tamil: Arunelli, Keela Nelli,
Malayalam: Arinelli, Kizhanelli, Nellipuli
Marathi: Rayavali, Bhuiavli,
Oriya: Narakoli
Sanskrit: Amala, Bhumyamlaki, Sukshmadala, Vitunika, Bhoodatri
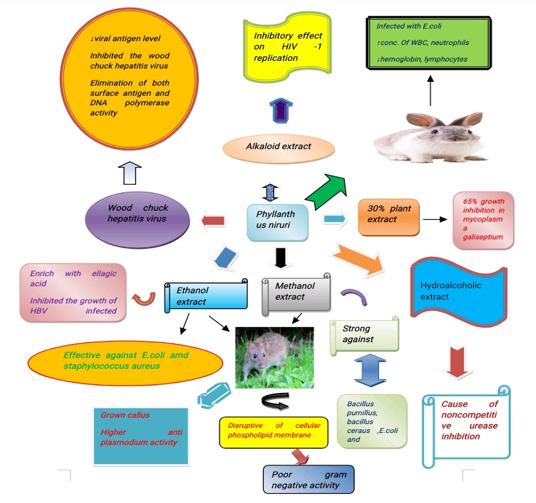
The extracts of P. niruri was tested against food borne & spoilage microorganisms. The growth of microorganisms was inhibited by the ethanolic extracts of P. niruri. [14] This extract is strong against Bacillus pumillus, Bacillus ceraus, E. coli and Vibrio cholera at conc. of 750 µg/ml/disc. The potency was increased by 49 % when the extract was fermented with lactobacillus. [15] It is also tested against the standard drug chloramphenicol at concentration of 10 µg/ml/disc shows potential source of antimicrobial agent. [16]
In vivo
The extract of alkaloids was tested on rabbits infected with E.coli. The results examined werefound to have increased concentration of WBC, neutrophils and decreased hemoglobin and lymphocytes but no changes in enzyme concentration [17].
In vitro
P. niruri can be used as a substitute of antibiotics in the treatment of Chronic Respiratory Disease (CRD) in broiler chickens caused by Mycoplasma galisepticum. A 30% plant extract caused up to 65% growth inhibition in Mycoplasma galisepticum.[18]
In a similar study conducted by Ramandeep and colleagues, they found that P. niruri extracts inhibits the growth of Escherichia coli (E. coli), Lactobacillus acidophilus (L. acidophilus), Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus). Hydroalcoholic extracts of P. niruri L was found to inhibit urease activity. Quercetin is one of the major constituents which is thought to be the cause of noncompetitive urease inhibition.[19]
Another study used silver nanoparticles (AgNPs) obtained from a supercritical CO2 extract of P. niruri to test the anti-bacterial potential against various strains like Coney lunata and Salmonella typhi.[20], [21]
These studies utilize different types of P. niruri extracts, with one study a comparison of methanolic, ethanolic and aqueous extracts, recognizing that different preparations yielded different compositions of pharmacophores. [22] An agar well diffusion study conducted on the antimicrobial activity of aqueous and ethanolic extracts of the leaves and roots of 4 Indian herbs, including P. niruri, showed that the ethanolic extract was more effective against Escherichia coli and Staphylococcus aureus, whereas the aqueous preparation had greater activity against Proteus vulgaris and Bacillus subtilis but poor anticoliform activity.[23]
The methanol extracts of P. niruri is twice strong as that of aqueous preparation. In addition, both aqueous and methanolic extracts of P. niruri demonstrated significant activity against Listeria monocytogenes, the bacteria responsible for listeriosis, suggesting the potential of P. niruri as a food preservative. [24] A subsequent disc diffusion study found that both ethanolic and aqueous extract of P. niruri failed to inhibit the growth of the Gram-negative bacilli but demonstrated statistically more significant inhibitory activity against Gram-positive bacteria. [25] The apparent difference in results between this study and that of Cheah and colleagues in 2011[24] could be due to the use of varying solvents.
This could suggest that the aqueous extracts contained a higher content of phenolic compounds compared with the ethanolic extract.[26] Agar diffusion assays in a study on Helicobacter pylori and three species of probiotic Lactobacilli revealed that P. niruri inhibited H. pylori in a dose-dependent manner while it did not affect the growth of lactic acid bacteria.[27]
The anti- H. pylori property of P. niruri did not involve the inhibition of proline dehydrogenase, a membrane-associated protein linked with prokaryotic energy production.[28] This may be due to the presence of ellagitannins such as geraniin and corilagin contained in the aqueous extract, which have also been to act in a concentration-dependent manner against various antibiotic-resistant H. pylori strains[29] by rapidly precipitating agglutination of H. pylori cells.[30]
In general, methanolic extracts are more potent against Gram-positive microbes, followed by aqueous extracts and ethanolic extracts. The antibacterial activity of P. niruri is also dose dependent. However, the compatibility of methanolic extracts to the mammalian subject may need to be investigated using animal models, as methanol, a polar organic solvent, may be disruptive of cellular phospholipid membranes. Although there is noticeably poor Gram-negative activity, there is still a need for large-scale molecular studies to investigate the relationship between the morphology of Gram-negative bacteria and the lack of Gram-negative activity in P. niruri extracts.
The in vitro anti-hepatitis B viral activity of P. niruri L. in Hep G2/C3A and SK-HEP-1 cells was studied by Li et al.[31] The ethanol fractions were analyzed and reported to be enriched with ellagic acid fractions which successfully inhibited the growth of HBV-infected HepG2 / C3A cells compared to the isolated active compound that showed a half-maximal inhibitory concentration (IC50) of 120 µg/mL and had no effect on HBV DNA replication at the concentrations evaluated, hence failing to inhibit the reproduction of HBV.[31] Hepatitis B virus claims around a million human lives annually. Sarma and colleagues attempted to explore a potent and efficient antiviral from Phyllanthus with a minimal risk of resistance for hepatitis B virus.[32] Moreover, in this attempt the Phyllanthus active principles from among 93 phytochemicals were isolated to check the mechanism of action against hepatitis B virus reverse transcriptase (HBV RT), which is an active target for drugs used against HBV infections.[32]
P. niruri is used as a home remedy for many diseases in Asia.[33] It is used to treat jaundice and to inhibit the hepadna virus[34] and duck hepatitis B virus by inhibiting 50 % of DNA polymerase.[35] Wood chuck hepatitis virus (WHV) was tested against the extract in wood chucks (Marmota monax), it efficiently inhibited the wood chuck hepatitis virus (WHV) and it eliminated of both surface antigen and DNA polymerase activity.[36]
Most prominent among the potential therapeutic effects of P. niruri is its antiviral activity. Studies conducted on sera obtained from chronic hepatitis B patients and woodchuck hepatitis (WHV)-infected woodchucks, which were treated with P. niruri extracts, showed decreased viral antigen levels.[37] Overall, aqueous extracts of P. niruri have been shown to possess significant antiviral potential and appear promising especially with regard to hepatitis B carriers.[36]
Although not all the bioagents responsible for the antihepatitis B activity of P. niruri have been identified, molecular studies have determined the molecular structure of a novel lignin found in P. niruri, nirtetralin B and its two stereoisomers, nirtetralin and nirtetralin A.
Nirtetralin
Nirtetralin significantly inhibited HBsAg and HBeAg levels in vitro. [38] All three lignans had a dose-dependent inhibitory effect on the in-vitro titres of HBV antigens. Moreover, when compared with acyclovir, inhibition ratios for nirtetralin and nirtetralin B were significant suggesting that these compounds are promising novel anti-HBV antivirals. These lignans had low cytotoxicity on host cells, suggesting that these compounds could safely be given at non-toxic dosages without incurring undesirable adverse drug reactions.[39], [40] Although, no systematic reviews have been conducted on the anti-hepatitis B activity of P. niruri, there have been a number of reviews since 2000, on the utility of Phyllanthus genus as potential antiviral agents for chronic hepatitis B infection.[41], [42], [43]
Despite the promising results with respect to the inhibition of HIV-1RT activity, repandusinic acid seemed to exert less significant inhibition of DNA polymerase alpha.[44] With regard to the resultant degree of cytopathogenicity, repandusinic acid reduced the number of pathogenic changes in HIV infected MT4 cells, and the results even suggested that repandusinic acid may be more potent than AZT in inhibiting HIV cytopathogenicity. Moreover, azidothymidine and repandusinic acid may work in synergy when administered as a combination. However, the action of repandusinic acid has only been studied at the cellular level, and no animal or human studies on the anti-HIV therapeutic effects of repandusinic acid have been carried out.
A study conducted by Ogata et al., noted that tannins of P. niruri such as repandusinic acid, as another novel HIV-1-RT inhibitor were important antiviral agents in HIV therapy.[44]
This significant toxic selectivity for virus infected cells was replicated in a subsequent study on alkaloidal extracts of P. niruri[45] with a greater preference for HIV-2-infected cell lines. Alkaloid extract of P. niruri also has an inhibitory effect on HIV infection.
Qian-Cutrone and colleagues isolated a glucopyranoside, niruriside, which was found to inhibit REV/RRE binding during the movement of viral RNA from the cell nucleus to the cytoplasm. However, despite being found to be a specific REV/RRE inhibitor, niruriside did not display satisfactory levels of cellular protection in cases of acute HIV-1 infection.[46]
A study exploring the antidengue activity of members of the genus Phyllanthus showed that Phyllanthus extractsworked best when administered simultaneously with DENV-2 inoculum implying that the Phyllanthus extract most probably affected the early phases of viral infection such as the viral attachment and entry.[47] Proteome analysis showed that the expression of 13 host and viral proteins involved in viral entry and replication, molecular chaperoning, cytoskeletal assembly and cellular metabolisms was altered, including calreticulin, Trim 1, heat-shock 70-kDA protein, beta-actin, DNA topoisomerase I, NS3, G3PD (glyceraldehyde-3-phosphate dehydrogenase), RBM1 (RNA-binding motif 1), DNA mismatch repair protein Msh2, dengue virus NS2bNS3 and polysialyl transferase.[47]
Apart from that, P. niruri-synthesized silver nanoparticles demonstrated significant larvicidal, pupicidal and adulticidal activity against Aedes aegypti both in laboratory and field settings.[48] Malaria is one of the most prominent health problems in the tropical and subtropical countries. The herbal plants show antagonistic properties against malaria. P. niruri and Mimosa pudica showed antiplasmodial activity, when feed with ethanol extracts in albino mice.[49] P. niruri’s ethanolic extract of one month old in vitro grown callus showed higher antiplasmodial activity than extract prepared from fresh apical stem extract.[50]
|
Extract |
Effect |
Part used |
References |
|
Ethanolic |
Inhibit the growth of microorganism |
Plant |
|
|
Alkaloids |
Increase concentration of WBC, neutrophils and decreased hemoglobin, lymphocytes |
Plant |
|
|
Alkaloids |
65% growth inhibition in mycoplasma galisepticum |
30% plant |
|
|
Hydro alcoholic |
Noncompetitive urease inhibition |
Plant |
|
|
Ethanolic |
Effective against E.coli, staphylococcus |
Root, leaves |
|
|
Ethanolic and aqueous |
Inhibitory activity against gram positive |
Plant |
|
|
Ethanolic |
Inhibited the growth of HBV – infected HepG2/C3A cells |
Plant |
|
|
Alkaloid |
Inhibitory effect on HIV -1 replication |
Plant |
Conclusion
Phyllanthus niruri belongs to a family Euphorbiaceae and it has many health benefits, one of the most important health benefits is antimicrobial activity. The anti-microbial activity has been studied in in vitro, in vivo and humans. In in vivo experiments on the rabbit it was found increased concentration of WBC, neutrophils and decreased hemoglobin, lymphocytes infected with an E.coli but there are no changes in enzyme concentration while given Phyllanthus niruri as a dose to overcome the antimicrobial activity in rabbit. In in vitro condition lactobacillus bacteria of curd as taken and studied even there, they have found the microbial activity has been reduced by incorporating the Phyllanthus niruri. Clinical studies on hepatitis B patients showed that 50–60% of patients who were administered P. niruri extract experienced HBsAg seroconversion. The reduction in HBsAg antigen may have been due to the inhibitory effect of P. niruri on hepatitis B viral genetic replication of note, in a study where patients were treated with extracts of three different members of the genus Phyllanthus, it was observed that extracts of P. niruri were more likely to induce reductions in HBeAg titers. Although not all the bioagents responsible for the antihepatitis B activity of P. niruri have been identified, molecular studies have determined the molecular structure of a novel lignin found in P. niruri, nirtetralin B and its two stereoisomers, nirtetralin and nirtetralin A are responsible.
Conflicts of Interest
All contributing authors declare no conflicts of interest.
Source of Funding
None.
References
- Lewis WH. Medicinal Plants as Sources of New Therapeutics. Ann Mo Bot Gard . 1995;82(1):16-24. [Google Scholar] [Crossref]
- Dhar ML, Dhar MM, Dhawan BN, Mehrotra BN, Srimal RC, Tandon JS. Screening of Indian plants for biological activity. IV. Indian J Exp Biol. 1973;11(1):43-54. [Google Scholar]
- Chauhan J, Sultan M, Srivastava S. TWO NEW GLYCOFLAVONES FROM THE ROOTS OF PHYLLANTHUS NIRURI. Planta Med. 1977;32(07):217-22. [Google Scholar] [Crossref]
- Ishimaru K, Yoshimatsu K, Yamakawa T, Kamada H, Shimomura K. Phenolic constituents in tissue cultures of Phyllanthus niruri. Phytochemistry. 1992;31(6):2015-8. [Google Scholar] [Crossref]
- Gupta DR, Ahmed B, Shoyakugaku Z. A new flavone glycoside from Phyllanthus niruri. J Nat Prod. 1984;383:213-5. [Google Scholar]
- Saija A, Tomaino A, Trombetta D, Pellegrino ML, Tita B, Messina C. In vitro' antioxidant and photoprotective properties and interaction with model membranes of three new quercetin esters. Eur J Pharm Biopharmaceutics: Official J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2003;56(2):167-74. [Google Scholar] [Crossref]
- Muzitano MF, Cruz EA, Almeida Ad, Silva SAD, Kaiser CR, Guette C. Quercitrin: An Antileishmanial Flavonoid Glycoside fromKalanchoe pinnata. Planta Med. 2006;72(1):81-3. [Google Scholar] [Crossref]
- Paithankar K, Ingle M. Influence of Parameters on Usability Attributes in Software Projects. Int J Adv Sci, Eng Inf Technol. 2011;1(3). [Google Scholar] [Crossref]
- Latté K, Kolodziej H. Antifungal Effects of Hydrolysable Tannins and Related Compounds on Dermatophytes, Mould Fungi and Yeasts. Zeitschrift für Naturforschung C. 2000;55(5-6):467-72. [Google Scholar] [Crossref]
- Hufford CD, Liu S, Clark AM. Antifungal Activity of Trillium grandiflorum Constituents. J Nat Prod. 1988;51(1):94-8. [Google Scholar] [Crossref]
- Mensah J, Lagarde I, Ceschin C, Michelb G, Gleye J, Fouraste I. Antibacterial activity of the leaves of Phyllanthus discoideus. J Ethnopharmacol. 1990;28(1):129-33. [Google Scholar] [Crossref]
- Subeki S, Matsuura H, Takahashi K, Yamasaki M, Yamato O, Maede Y. Anti-babesial and Anti-plasmodial Compounds fromPhyllanthusniruri. J Nat Prod. 2005;68(4):537-9. [Google Scholar] [Crossref]
- Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals fromPhyllanthus niruriLinn. and their pharmacological properties: a review. J Pharm Pharmacol. 2006;58(12):1559-70. [Google Scholar] [Crossref]
- Lopez CL, Nitisinprasert S. Antimicrobial Activity of Medicinal Plant Extracts against Foodborne Spoilage and Pathogenic Microorganisms. J Kas Nat Sci. 2003;37:460-7. [Google Scholar]
- Venugopalan V, Dinesh MS, Geetha KS. Enhancement of antimicrobial potential of phyllanthus niruri by fermentation. J Herb Med Toxi. 2010;4(2):167-75. [Google Scholar]
- Yerra A, Sunder JS. Malaya gupta and upal kanti mazumder. Vitro Lipid Peroxidation Inhibitory and antimicrobial Activity of Phyllanthus niruri. 2008;7:67-70. [Google Scholar]
- Ajibade VA, Egbebi AO. Effect of alkaloid extract of phyllanthus niruri on rabbits infected with enteropathogenic Escherichia coli. Int J Trop Med Pub Health. 2011;1(1). [Google Scholar]
- Sabdoningrum EK, Hidanah S, Wahjuni R, Chusniati S, Arimbi A. An In Vitro Antibacterial Activity Test of Meniran Herbs’(Phyllanthus Niruri L.) Ethanol ExtractAgainst Mycoplasma gallisepticum causes Chronic Respiratory Disease (CRD) in Broiler Chickens. KnE Life Sci. 2017;3(6):48-61. [Google Scholar] [Crossref]
- Kaur B, Kaur N, Gautam V. Evaluation of anti-helicobacter pylori (DSMZ 10242) activity and qualitative analysis of quercetin by HPLC in Phyllanthus niruri linn. World J Pharm Pharm Sci. 2016;5:1691-706. [Google Scholar]
- Haris M, Kumar A, Ahmad A, Abuzinadah MF, Basheikh M, Khan SA. Microwave-assisted green synthesis and antimicrobial activity of silver nanoparticles derived from a supercritical carbon dioxide extract of the fresh aerial parts of Phyllanthus niruri L. Trop J Pharm Res. 2018;16(12). [Google Scholar] [Crossref]
- Ahamath JM, Vahith RA, Manivel V, Elamparithi R. Phytochemical Screening and Antimicrobial Activity of Phyllanthus niruri. J Adv Appl Sci Res. 2017;1. [Google Scholar]
- Shanmugam B. Antibacterial activity and phytochemical screening of Phyllanthus niruri in ethanolic, methanolic and aqueous extracts. Int J Pharmaceut Sci Rev Res. 2014;27:85-9. [Google Scholar]
- Chitravadivu C. Antimicrobial studies on selected medicinal plants, Erode region. J Sci Res. 2009;4:147-52. [Google Scholar]
- Poh-Hwa T. Bioprotective properties of three Malaysia Phyllanthus species: an investigation of the antioxidant and antimicrobial activities. Int Food Res J. 2011;18:887-93. [Google Scholar]
- Amin ZA, Abdulla MA, Ali HM, Alshawsh MA, Qadir SW. Assessment of In vitro antioxidant, antibacterial and immune activation potentials of aqueous and ethanol extracts of Phyllanthus niruri. J Sci Food Agriculture. 2012;92(9):1874-7. [Google Scholar] [Crossref]
- Ibrahim D, Hong LS, Kuppan N. Antimicrobial Activity of Crude Methanolic Extract from Phyllanthus niruri. Nat Prod Commun. 2013;8(4):493-6. [Google Scholar] [Crossref]
- Ranilla L, Apostolidis E, Shetty K. Antimicrobial Activity of an Amazon Medicinal Plant (Chancapiedra) (Phyllanthus niruri L.) against Helicobacter pylori and Lactic Acid Bacteria. Phytotherapy Res. 2012;26(6):791-9. [Google Scholar] [Crossref]
- Lin YT, Kwon YI, Labbe RG, Shetty K. Inhibition of Helicobacter pylori and Associated Urease by Oregano and Cranberry Phytochemical Synergies. Appl Environ Microbiol. 2005;71(12):8558-64. [Google Scholar] [Crossref]
- Voravuthikunchai SP, Mitchell H. Inhibitory and Killing Activities of Medicinal Plants against Multiple Antibiotic-resistant Helicobacter pylori. J Health Sci . 2008;54(1):81-8. [Google Scholar] [Crossref]
- Funatogawa K, Hayashi S, Shimomura H, Yoshida T, Hatano T, Ito H. Antibacterial Activity of Hydrolyzable Tannins Derived from Medicinal Plants againstHelicobacter pylori. Microbiol Immunol. 2004;48(4):251-61. [Google Scholar] [Crossref]
- Li Y, Li X, Wang J, Kuang Y, Qi M. Anti-hepatitis B viral activity of Phyllanthus niruri L. (Phyllanthaceae) in HepG2/C3A and SK-HEP-1 cells. Trop J Pharm Res. 2017;16:1873-9. [Google Scholar]
- Sarma K, Borkakoty B, Parida P, Jakharia A, Dey D, Biswas D. In Silico Identification of Natural Lead Molecules from the Genus of Phyllanthus Against Hepatitis B Virus Reverse Transcriptase. Nat Prod J. 2016;6(4):292-304. [Google Scholar] [Crossref]
- Thyagarajan S, Thirunalasundari T, Subramanian S, Venkateswaran P, Blumberg B. Effect of Phyllanthus amarus on chronic carriers of hepatitis B virus. Lancet. 1988;332(8614):764-6. [Google Scholar] [Crossref]
- Unander DW, Webster GL, Blumberg BS. Usage and bioassays in Phyllanthus (Euphorbiaceae). IV. Clustering of antiviral uses and other effects. J Ethnopharmacol. 1995;45(1):1-18. [Google Scholar] [Crossref]
- Shead A, Vickery K, Pajkos A, Medhurst R, Freiman J, Dixon R. Effects of Phyllanthus plant extracts on duck hepatitis B virus in vitro and in vivo. Antivir Res. 1992;18(2):127-38. [Google Scholar] [Crossref]
- Venkateswaran PS, Millman I, Blumberg BS. Effects of an extract from Phyllanthus niruri on hepatitis B and woodchuck hepatitis viruses: in vitro and in vivo studies.. Proce Natl Acad Sci. 1987;84(1):274-8. [Google Scholar] [Crossref]
- Thyagarajan SP, Thiruneelakantan K, Subramanian S, Sundaravelu T. In vitro inactivation of HBsAg by Eclipta alba Hassk and Phyllanthus niruri Linn. Indian J Med Res. 1982;76:124-30. [Google Scholar]
- Huang RL, Huang YL, Ou JC, Chen CC, Hsu FL, Chang C. Screening of 25 compounds isolated from Phyllanthus species for anti-human hepatitis B virus in vitro. Phytotherapy Res. 2003;17(5):449-53. [Google Scholar] [Crossref]
- Liu S, Wei W, Li Y, Lin X, Shi K, Cao X. In vitro and in vivo anti-hepatitis B virus activities of the lignan nirtetralin B isolated from Phyllanthus niruri L. J Ethnopharmacol. 2014;157:62-8. [Google Scholar] [Crossref]
- Liu S, Wei W, Shi K, Cao X, Zhou M, Liu Z. In vitro and in vivo anti-hepatitis B virus activities of the lignan niranthin isolated from Phyllanthus niruri L. J Ethnopharmacol. 2014;155(2):1061-7. [Google Scholar]
- Liu J, Lin H, McIntosh H. Genus Phyllanthus for chronic hepatitis B virus infection: a systematic review. J Viral Hepatitis. 2001;8(5):358-66. [Google Scholar] [Crossref]
- Xia Y, Luo H, Liu JP, Gluud C. Phyllanthus species for chronic hepatitis B virus infection. . Cochrane Database Syst Rev. 2011. [Google Scholar] [Crossref]
- Xia Y, Luo H, Liu JP, Gluud C. Phyllanthus species versus antiviral drugs for chronic hepatitis B virus infection. . Cochrane Database Syst Rev. 2013. [Google Scholar] [Crossref]
- TO, Higuchi H, Mochida S, Matsumoto H, Kato A, Endo T. HIV-1 Reverse Transcriptase Inhibitor from Phyllanthus niruri. AIDS Res Human Retroviruses. 1992;8(11):1937-44. [Google Scholar] [Crossref]
- Naik AD, Juvekar AR. Effects of alkaloidal extract of Phyllanthus niruri on HIV replication. Indian J Med Sci. 2003;57(9):387-93. [Google Scholar]
- Qian-Cutrone J, Huang S, Trimble J, HLi, Lin PF, Alam M. Niruriside, a New HIV REV/RRE Binding Inhibitor fromPhyllanthus niruri. J Nat Prod. 1996;59(2):196-9. [Google Scholar] [Crossref]
- Lee SH, Tang YQ, Rathkrishnan A, Wang S, Ong KC, Manikam R. Effects of cocktail of four local Malaysian medicinal plants (Phyllanthus spp.) against dengue virus 2. BMC Complement Altern Med. 2013;13. [Google Scholar] [Crossref]
- Suresh U, Murugan K, Benelli G, Nicoletti M, Barnard DR, Panneerselvam C. Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol Res. 2015;114(4):1551-62. [Google Scholar] [Crossref]
- Arthi N. Anti malarial activity and phytochemical screening of ethanolic leaf extract of phyllanthus niruri and mimosa pudica. IJPRD. 2011;3(3):198-205. [Google Scholar]
- Cimanga R, Tona L, Luyindula N, Mesia K, Lusakibanza M, Musuamba C. In vitro antiplasmodial activity of callus culture extracts and fractions from fresh apical stems of Phyllanthus niruri L. (Euphorbiaceae): part 2. J Ethnopharmacol. 2004;95(2-3):399-404. [Google Scholar] [Crossref]
How to Cite This Article
Vancouver
G C, R M, S V, R SN, MS M. Antimicrobial activity of Phyllanthus niruri (Chanka piedra) [Internet]. J Nutr Metab Health Sci. 2020 [cited 2025 Nov 02];3(4):103-108. Available from: https://doi.org/10.18231/j.ijnmhs.2020.021
APA
G, C., R, M., S, V., R, S. N., MS, M. (2020). Antimicrobial activity of Phyllanthus niruri (Chanka piedra). J Nutr Metab Health Sci, 3(4), 103-108. https://doi.org/10.18231/j.ijnmhs.2020.021
MLA
G, Chandana, R, Manasa, S, Vishwanath, R, Shekhara Naik, MS, Mahesh. "Antimicrobial activity of Phyllanthus niruri (Chanka piedra)." J Nutr Metab Health Sci, vol. 3, no. 4, 2020, pp. 103-108. https://doi.org/10.18231/j.ijnmhs.2020.021
Chicago
G, C., R, M., S, V., R, S. N., MS, M.. "Antimicrobial activity of Phyllanthus niruri (Chanka piedra)." J Nutr Metab Health Sci 3, no. 4 (2020): 103-108. https://doi.org/10.18231/j.ijnmhs.2020.021
